- Products ({{ products.totalElements }})
- News & articles ({{ wpNewsAndArticles.total_posts }})
- Blog entries ({{ wpBlogEntries.total_posts }})
|
{{ String(product.attributes.name) }}
{{ String(product.attributes.productType) }}
-
NEW
|
{{ String(product.attributes.artNo) }} | {{ (String(product.attributes.sizeStr) != 'undefined') ? String(product.attributes.sizeStr) : '–' }} |
Not available
|
| No products found |
Optibodies™ - When Good Is Not Enough

Optibodies™ – A series of monoclonal antibodies for immunohistochemistry
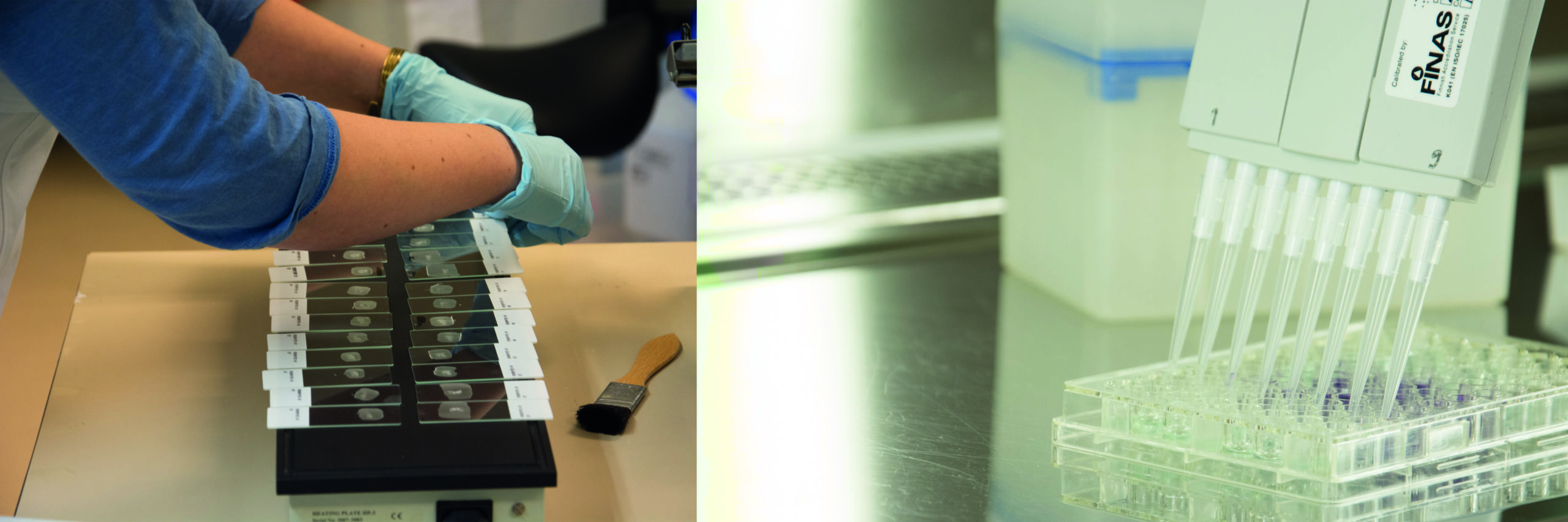
Optibodies™ are a series of monoclonal antibodies for immunohistochemistry manufactured by Nordic BioSite. They were created to boost quality and create options for users of IHC biomarkers in clinical pathology. High quality is the driving force, only antibodies that reach excellent results in testing protocol and appropriate control tissues are approved as part of the collection. An important aspect is being user-friendly, Optibodies™ works for different protocols and platforms with standard buffers. Optibodies™ get high scores in NordiQC assessments which is a confirmation of the high quality.
Are all Optibodies CE/IVD labeled?
Yes, all Optibodies are CE/IVD
Do you use external control for the Optibodies performance?
Yes, the Optibodies are evaluated by external partners and also participate in NordiQC
Which pretreatment is recommended for Optibodies?
All IHC-P protocols for Optibodies are for HIER in standard pH 9 buffer
What dilution is recommended for Optibodies?
1:100 to 1:400 is the range for Optibodies. The dilution depends on the platform and the staining system used and should always be determined by the user.
Do you have experience with the use of Optibodies on other species than human?
Yes. Please inquire.
Would you be interested in testing out Optibodies™ in your own lab, please contact us at info@nordicbiosite.com and we would be happy to discuss the options with you. Protocols for IHC automation platforms are available on the Optibodies™ product page.
Get more knowledge about Optibodies™ in our ScienceHub! Read the latest blog post and news.
How antibodies are optimized into Optibodies™
The main points when optimizing an antibody are selecting the correct tissues, which are appropriately fixed and processed, and of course, selecting the correct primary antibody. Optimization of antibodies should always be performed with more than one antibody against the same tissue antigen to get results for comparison. Different pretreatment methods are important to test as well as fine-tuning antibody concentration and incubation times. From a clinical perspective, antibodies must be specific with a high affinity towards their epitopes. Of course, they also have to be flexible and reliable to use and have a good Lot-to-Lot-consistency.
The top-performing Optibodies™
We want you to experience the high quality of Optibodies™ and we want to make it easy for you. Check out the images and the protocols for Desmin, MSH6, Pan Cytokeratin, EpCAM, and Napsin A, all CE/IVD marked. We have protocols for Ventana Benchmark Ultra and Dako Omnis if you want to test them in your own lab.
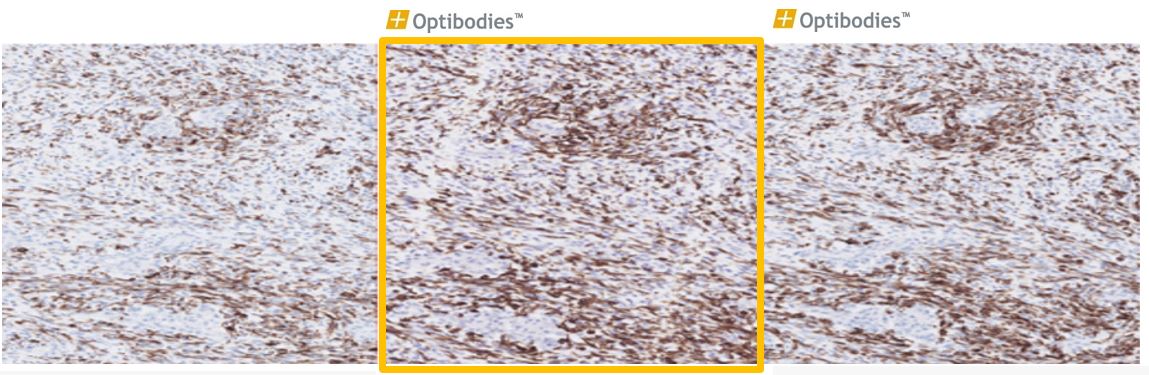
Desmin(BS21)
Desmin is an intermediate filament expressed in smooth and skeletal muscle cells and used as a marker to distinguish tumors of myogenic origin. The Desmin antibody (clone BS21) has great analytical and technical sensitivity.
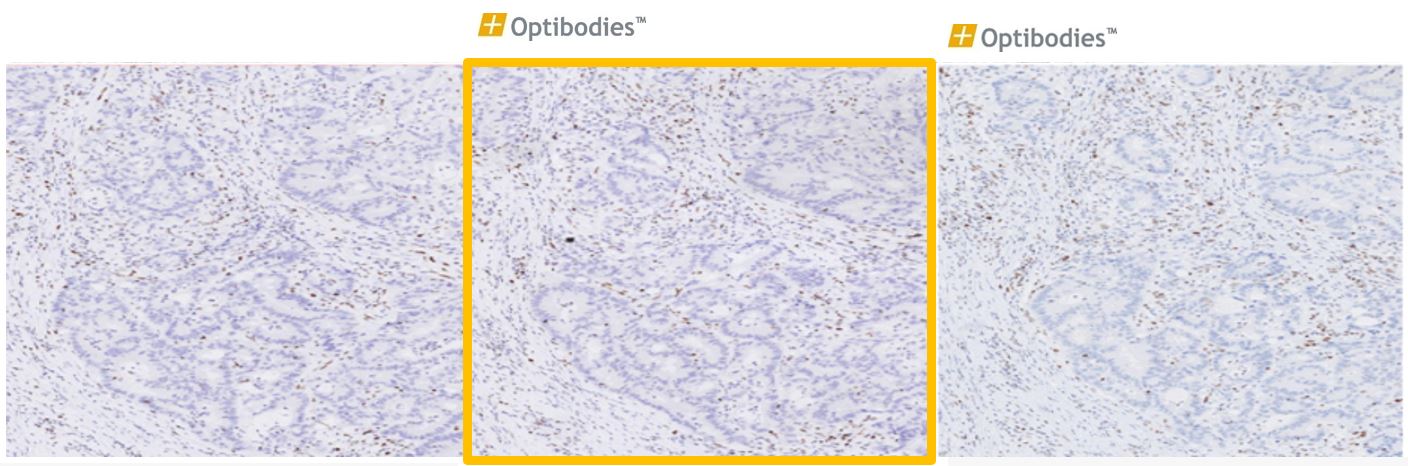
MSH6 (BSR100)
Colon Adenocarcinoma
MSH6 (MutS Homolog 6) is a member of DNA mismatch repair system and it is associated with certain colon cancer, colorectal cancer, and endometrial cancer. Loss of MSH6 expression can be visualized in these cancer types by the MSH6 antibody (clone BSR100)
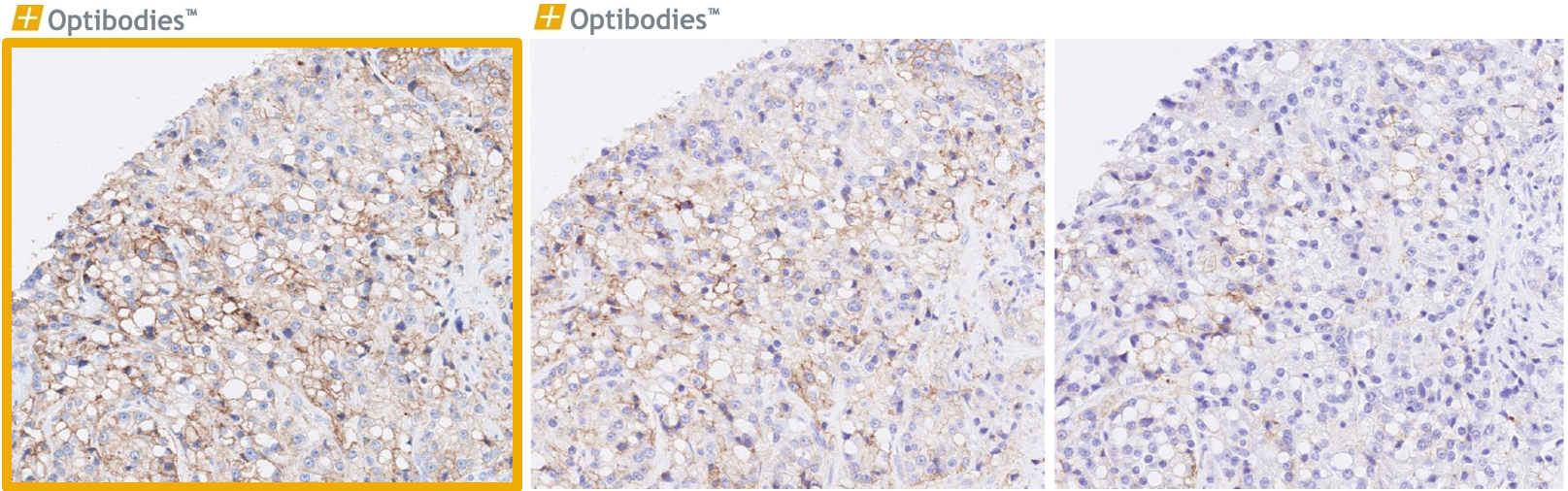
Pan Cytokeratin (BS5)
Cytokeratins are expressed in a wide variety of epithelial tissues and are of great importance for carcinoma diagnostic. The challenge is to stain all the 20 different cytokeratins optimally. BS5 clone meets this challenge as one of the best clones on the market. The main advantage of the clone is the monoclonality, providing robust performance independent of the tissue and between many protocols.
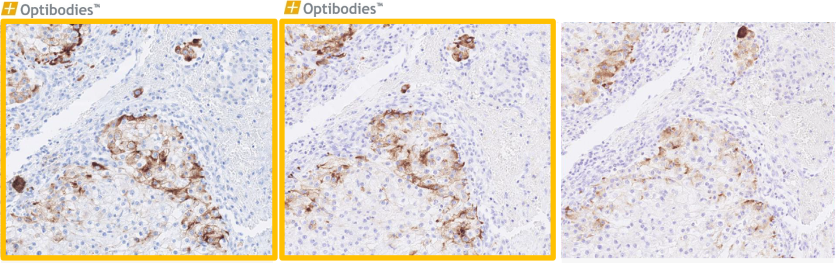
EpCAM (BS14)
Ep-CAM, is an epithelial marker and can be of great help in the differential diagnosis of malignancies in the peritoneal and pleural cavities. The advantage of the BS14 clone is its robustness and optimal performance on multiple IHC platforms, using standard high pH HIER buffer. The BS14 clone is a good alternative with a more flexible dilution range.
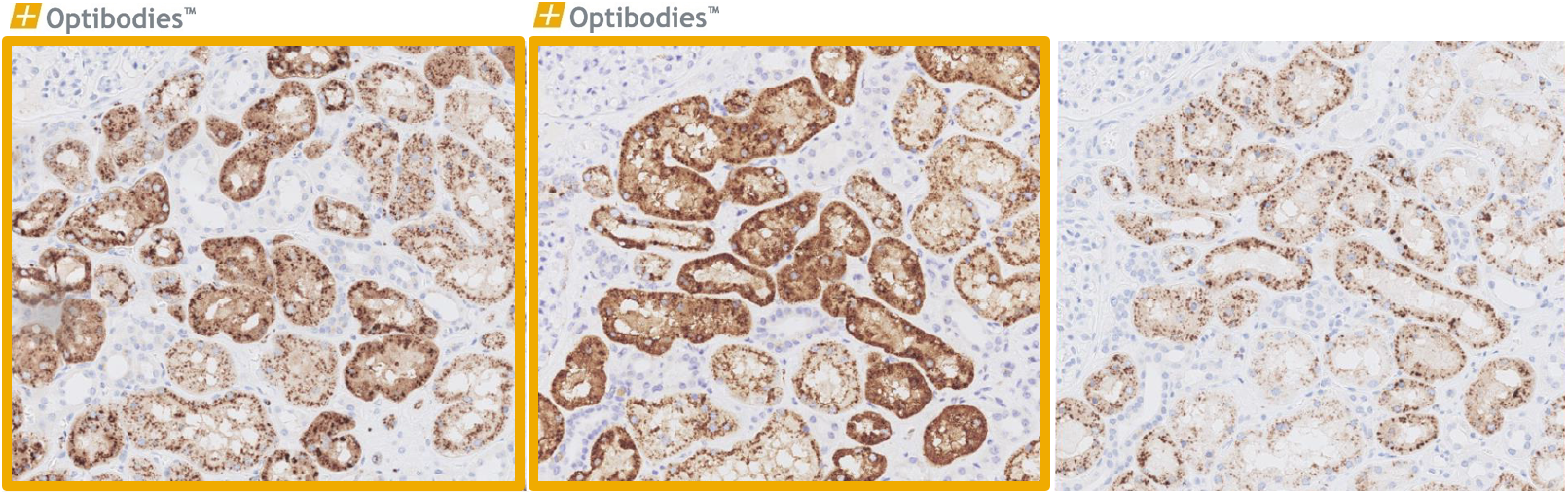
Napsin A (BS10)
Napsin A is predominantly expressed in the lung and kidney. It is important in the differential diagnosis of lung adenocarcinoma vs. squamous cell carcinoma. The main advantage of BS10 is its excellent performance, being sensitive and specific as well easy to optimize.